What Major Changes Occur in the Circulation of Blood in the Heart of a Newborn Baby?
Abstruse
The transition to newborn life at birth involves major cardiovascular changes that are triggered by lung aeration. These include a large increase in pulmonary blood flow (PBF), which is required for pulmonary gas exchange and to supervene upon umbilical venous return as the source of preload for the left eye. Clamping the umbilical cord before PBF increases reduces venous render and preload for the left middle and thereby reduces cardiac output. Thus, if ventilation onset is delayed following cord clamping, the infant is at risk of superimposing an ischemic insult, due to depression cardiac output, on top of an asphyxic insult. Much debate has centered on the timing of cord clamping at birth, focusing mainly on the potential for a time-dependent placental to infant claret transfusion. This has prompted recommendations for delayed cord clamping for a set up time after birth in infants not requiring resuscitation. All the same, recent bear witness indicates that ventilation onset earlier cord clamping mitigates the adverse cardiovascular consequences caused by immediate cord clamping. This indicates that the timing of cord clamping should be based on the infant's physiology rather than an arbitrary flow of fourth dimension and that delayed cord clamping may be of greatest benefit to apneic infants.
Master
The transition from fetal to newborn life at birth represents a major physiological challenge that all humans must undertake to survive. Before birth the future airways of the lungs are liquid-filled and the lungs accept no office in gas exchange, which instead occurs across the placenta (ane). In addition, pulmonary blood menses (PBF) is low because pulmonary vascular resistance (PVR) is high, redirecting the majority of correct ventricular output (RVO) through the ductus arteriosus (DA) and into the systemic circulation (2,iii). While this physiological system provides the fetus with key adaptive advantages that permit it to survive and flourish in utero, they are not conducive to survival later on birth. In particular, separating the infant from the placenta (the fetal organ of gas exchange) by clamping the umbilical cord necessitates the rapid switch to pulmonary gas exchange within minutes of nativity. This switch not only involves aeration of the airways and gas-substitution regions of the lung, merely also includes a major reorganization of the fetal cardiovascular system (two,3,4). Specifically, PVR must decrease rapidly and then that PBF can increase and become the sole recipient of RVO. This is not but critical for ensuring that the lung's gas-commutation efficiency is adequate, but too critical for increasing PBF and enabling it to go the sole source of preload for the left ventricle (5,half-dozen). This is because PBF is the only source of venous return for the left center in adults and so PBF must accept the capacity to replace umbilical venous return as the primary source of preload for the left ventricle when the cord is clamped. As all of these events must occur inside minutes of birth and all are critical for survival, information technology is not surprising that they are linked and mostly triggered by a single event: lung aeration (5,6). Lung aeration triggers the subtract in PVR and increase in PBF, which, combined with umbilical string clamping, initiates a sequence of changes that dramatically reorganize the infant's circulation. As a result, major vascular shunts close, leading to anatomical separation of the pulmonary and systemic circulations and right and left sides of the heart, transforming the newborn'southward circulation from a fetal circuit into the adult phenotype (2).
Such is the importance of these changes that much research has focused on the circulatory transition at birth. However, until recently the inter-relationships between lung aeration, the increase in PBF, umbilical cord clamping, and flow reversal through the DA have not been examined (5). In item, the changes have not been recognized equally an integrated sequence of events that are highly interdependent and may significantly impact on the infant's cardiovascular part in the newborn flow. This review will focus on recent developments that have advanced our understanding of how the infant transitions at birth and have provided new insights into improving clinical practice, such as the advisable timing for umbilical cord clamping.
Lung Aeration "Triggers" The Increase in Pulmonary Blood catamenia at Nascence
By maintaining the lungs in a distended land, the presence of liquid within the hereafter airways during fetal life plays an essential part in lung development (1). However, at birth the airways must be cleared of this liquid to allow the entry of air and the onset of pulmonary gas exchange. Recent X-ray imaging studies accept shown that afterwards birth, airway liquid clearance predominantly occurs due to inspiratory activity. This creates a hydrostatic pressure gradient that facilitates the movement of liquid from the airways into the surrounding tissue (7,eight,9). Equally a consequence, liquid accumulates within the interstitial tissue compartment forming perivascular fluid cuffs (10), which increases pressure within the lung's interstitial tissue compartment (11). As liquid is cleared from the airways (typically within the first three–5 breaths) much more than quickly than from lung tissue (about 4 h), the breast wall must aggrandize to adapt the increment in lung book acquired by the increment in resting lung air volume (five,7,12). This highlights the importance of the infant having a compliant breast wall at nascence during the transition. In the absence of a compliant chest wall, failure of the chest wall to expand would increment intrathoracic pressures and interfere with the baby's respiratory and cardiovascular systems, the latter through reducing venous render and increasing PVR.
Although lung aeration is accepted as the primary trigger for the increase in PBF at birth ( Figure ane ) (four), what remain less certain are the mechanisms past which aeration exerts this effect. A number of mechanisms are likely to be involved including increased oxygenation, enhanced activity of vasodilator agents, and a multifariousness of mechanical effects associated with lung aeration (3,thirteen). These include a "mechanical outcome" of ventilation and changes in alveolar/capillary transmural pressures resulting from the formation of surface tension, which increases lung recoil despite the presence of surfactant (14,fifteen). These topics have been covered in an excellent recent review (3) so will not be discussed in detail here. Instead, we will focus on more recent insights, which propose that a key component of the sequence of events leading to the decrease in PVR and increase in PBF at nativity may have gone largely unnoticed.
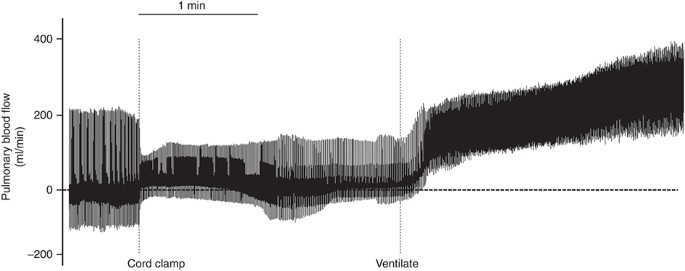
Instantaneous pulmonary claret menses (PBF) measured earlier, during, and later on umbilical string clamping (cord clamp) and after ventilation onset (ventilate); each spike represents a unmarried heartbeat. Note that before cord clamping, PBF oscillates effectually zero during the cardiac wheel, with maximum values indicating elevation systolic flows and minimum values indicating flows during diastole. Positive PBF values indicate antegrade period into the lungs whereas negative values indicate retrograde catamenia away from the lungs. While cord clamping markedly alters the PBF amplitude, which is due to a reduction in blood volume ejected per heartbeat, ventilation onset quickly and markedly increases PBF, resulting in forward catamenia into the lungs throughout the cardiac cycle.
PowerPoint slide
The rationale underpinning all of the proposed mechanisms responsible for the increase in PBF at birth suggests that the response is solely activated by air entry (four), which presumably initiates vasodilation in aerated regions in a locally dependent manner. This assumption is based on a number of facts as well equally data extrapolated from adult physiology. For instance, increasing and decreasing fetal oxygenation are well known (3) to increment and decrease PBF, respectively, independently of other factors. Similarly, i.v. assistants of vasodilators can vasodilate the pulmonary vascular bed and increase PBF (3,xiii), although this vasodilation cannot be sustained. While this shows that the perinatal lung is responsive to oxygen and vasodilators, information technology also indicates that air entry must cause irreversible changes that act to alter the signaling pathways for cardinal vasoactive agents causing localized vasodilation (iii). The latter is an extrapolation from the known ventilation/perfusion relationships in adult lung whereby increased alveolar oxygen levels cause localized vasodilation through nitric oxide-mediated pathways (16). This increases blood flow to well-ventilated lung regions ensuring appropriate matching of ventilation and blood menstruum. In addition, the introduction of air into the lungs causes surface tension to course and increase lung recoil in a locally dependent manner (15,17). The increase in alveolar wall recoil increases capillary/alveolar wall transmural pressures, which increases capillary caliber in aerated regions (4). Alterations in alveolar/capillary wall transmural pressures are a well-established determinant of pulmonary capillary menses and are a major determinant of vascular resistance inside the mature lung (18,19). The latter is responsible for the well-described vertical distribution of capillary blood catamenia within the developed lung and alveolar capillary recruitment that markedly reduces PVR with increasing cardiac output.
Based on this knowledge, the spatial relationship between regional lung aeration and regional changes in PBF has recently been examined with the expectation that regional lung aeration would increment PBF only in aerated lung regions (20). This was investigated using simultaneous stage contrast X-ray imaging and angiography, which uses the power of imaging to identify the spatial relationships between aeration and blood catamenia ( Effigy 2 ). Contrary to what was expected, regional lung aeration triggered a global increase in PBF, indicating that the increase in PBF was not spatially related to lung aeration (xx). This observation alone indicates that our electric current understanding for how lung aeration stimulates an increase in PBF requires re-evaluation. Indeed, information technology is possible that an unknown machinery, possibly contained of oxygen and the other mechanisms known to regulate regional PBF, triggers the initial increase in PBF (twenty). Whatsoever the mechanism(s), it is clear that a major ventilation perfusion mismatch occurs in the lung at birth when only a portion of the lung aerates ( Figure ii ). This is of import because regionalized aeration is a mutual event when initiating ventilation in very preterm infants. To compensate, the caregiver usually increases the oxygen pressure slope by increasing the inspired oxygen content. However, it is possible that this "spatial disconnection" betwixt lung aeration and increased PBF at nativity has adaptive advantages. This is because the increment in PBF is key to maintaining cardiac output after nascence, by becoming the sole source of left ventricular preload (see below). Although optimizing pulmonary oxygen exchange in the firsthand newborn flow is important, maintaining cardiac office is fifty-fifty more important to avoid ischemia contributing to any hypoxic events. If correct, and then it would seem preferable to ensure that the increase in PBF and thus left ventricular preload is not limited by an inability to completely aerate the lung at birth. Clearly, further studies are required to decide how lung aeration triggers an increase in PBF in a spatially independent manner and the relative roles of systemic oxygenation and circulating vasodilators.
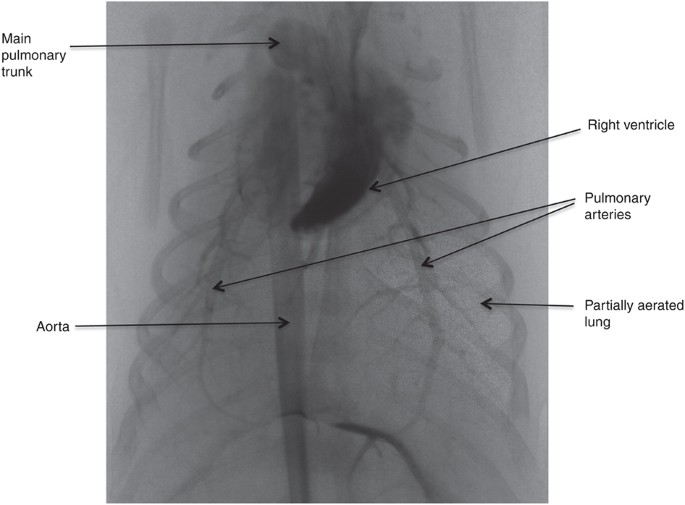
Simultaneous phase dissimilarity and angiographic 10-ray image of a virtually-term rabbit kitten following fractional ventilation of the right lung (20). Aerated lung regions tin can be seen as "speckle" in the epitome and is due to refraction at the air/h2o interface (8,34). The pulmonary blood vessels are highlighted past the injection of an iodine dissimilarity amanuensis, which also highlights the right ventricle and the aorta. Despite aeration of only ane lung, pulmonary blood flow (PBF) (as indicated past the amount of iodine contrast agent) was observed to increment in both aerated and unaerated lung regions (20).
PowerPoint slide
Cardiovascular Changes at Nativity: Event of Cord Clamping
In comparison to adults, the fetal circulatory organisation is considerably more than circuitous. Considering PVR is high, the bulk of RVO bypasses the lungs and flows through the DA (right-to-left shunting), passing direct into the descending thoracic aorta. Much of this output then passes through the placental circulation for, among other things, oxygenation (2,3). As the placental apportionment receives a loftier proportion of total fetal cardiac output (30–50% depending on species and gestational age), it is also a large source of venous return and preload for the fetal heart (two). In fetal sheep, at least l% of umbilical venous return passes through the ductus venosus and, without meaning mixing in the inferior vena cava, passes directly into the left atrium through the foramen ovale (2). Equally a result, umbilical venous return is the main source of preload for the left ventricle, particularly equally fetal PBF is very low and is unable to provide sufficient venous return to maintain left ventricular output (ii). The finding of a close inverse human relationship between PBF and menstruation through the foramen ovale (FO) in human fetuses indicates that the provision of left ventricular preload is not static, but is dependent upon a competitive inter-relationship betwixt these 2 sources of venous return (21). Although information technology is mostly assumed that fetal PBF is perpetually depression, based on early fetal studies (two), nosotros now know that this interpretation is non entirely accurate. Indeed, PBF markedly increases (up to fourfold) during fetal animate movements (FBMs), which is thought to exist due to dynamic and transient (breath-by-jiff) increases in the capillary/interstitial tissue transmural pressures, leading to a transient decrease in PVR (22). As such, it is likely that the relative contribution of these two sources of preload (PBF and flow through the FO) will vary depending upon factors such equally fetal activity.
In the fetus, the presence of the DA and the loftier PVR confers a unique characteristic pattern to the PBF waveform (22,23), which can be influenced by fetal action such as FBMs (22). During systole, blood flows toward the lungs but during late systole and throughout most of diastole, claret flows retrogradely in the pulmonary arteries away from the lungs ( Figure iii ). This retrograde flow is due to blood reflecting off the highly constricted pulmonary vascular bed and exiting the pulmonary circulation by flowing through the DA and entering the systemic circulation (24). As a result, blood flows continuously in the DA throughout the cardiac cycle with relatively high basal flows during diastole (23). As menstruation in the primary pulmonary trunk, which is just 1–2 cm upstream of the DA, decreases to zip during diastole (ii), the high basal flows in the DA during diastole are entirely attributable to retrograde flow in the left and right pulmonary arteries ( Effigy three ).
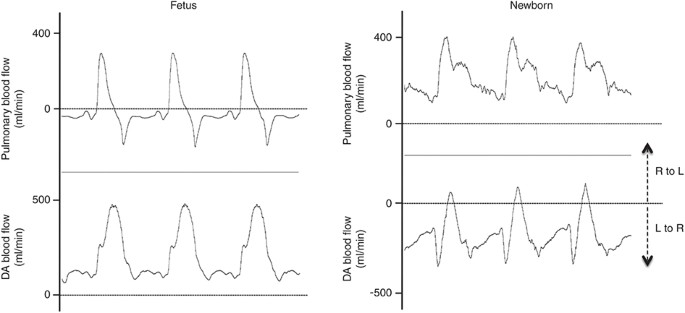
Blood flow waveforms in the left pulmonary artery and ductus arteriosus (DA) throughout three consecutive heartbeats in a lamb before (Fetus) and after (Newborn) ventilation onset. Note the shift in the blood period waveforms in relation to zero menstruum earlier and later on ventilation onset. Pulmonary blood flow (PBF) oscillates around nil before ventilation onset (negative flows reflect retrograde flow of blood away from the lungs), merely is positive throughout the cardiac cycle after ventilation onset. Earlier ventilation onset, flow in the DA is positive throughout the cardiac wheel. This indicates that blood flows right-to-left (R to L), from the pulmonary apportionment into the aorta, continuously throughout the cardiac cycle. R to L DA flow during diastole before ventilation onset is due to retrograde PBF. Post-obit ventilation onset, DA claret catamenia is predominantly left-to-right (L to R), flowing from the aorta and into the lungs, throughout about of the cardiac bike, except during early systole. Fifty to R period in the DA post-obit ventilation onset significantly contributes to PBF (23) and is entirely responsible for PBF during diastole.
PowerPoint slide
Cardiovascular Changes with Firsthand Umbilical Cord Clamping
At nascency, immediate clamping of the umbilical cord reduces venous return to the heart by 30–l% and instantaneously increases systemic vascular resistance by removing the low-resistance placental circulation from the systemic circuit (25). As a upshot, arterial pressure rapidly increases by about thirty% inside iv heartbeats and cardiac output decreases by 30–fifty%. The latter is due to both the increase in afterload and the decrease in venous render (which decreases preload) (25). In improver, cerebral blood flow initially increases, presumably due to the rapid increase in arterial pressure level, but then chop-chop decreases over again as cardiac output decreases and arterial blood pressures stabilize. Cardiac output then remains low until ventilation commences and PBF increases, which restores venous return and left ventricular preload earlier restoring cardiac output ( Effigy 4 ). The relative timing of cord clamping and lung aeration are therefore critical to a smooth transition and, if out of sequence, may expose the newborn to significant cardiovascular instability leading to complications including hemorrhage.
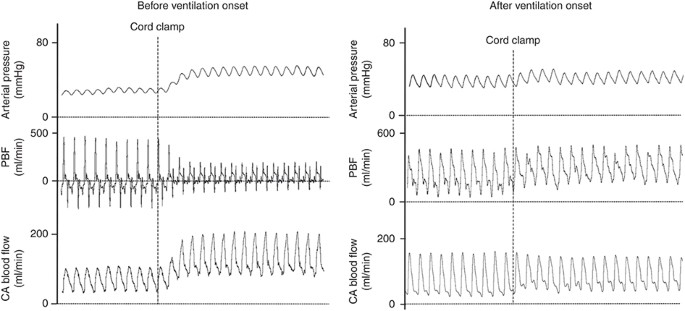
Issue of umbilical cord clamping (string clamping) on systemic arterial pressure (carotid artery), pulmonary blood menstruum (PBF), and carotid arterial (CA) claret flow measured in newborn lambs earlier or after ventilation onset. Note that if cord clamping occurs later on ventilation onset, the increases in CA pressure and blood menses are greatly mitigated as is the decrease in right ventricular stroke volume, indicated by maintained aamplitude in PBF waveform. The reduced increment in CA force per unit area is because the pulmonary apportionment, due to left-to-right shunting through the ductus arteriosus (DA), can immediately act as an alternate depression-resistance pathway for blood flow emanating from the left ventricle (25).
PowerPoint slide
Due to the combined effect of a decrease in PVR and an increase in systemic vascular resistance caused by umbilical string clamping, cyberspace flow through the DA reverses resulting in net left-to-right shunting across the DA (23). As a result, flow through the DA significantly contributes to the increase in PBF immediately after birth, which causes a major modify in the PBF waveform and results in a significant left ventricle, lung, left ventricle short circuit. Retrograde PBF is rapidly abolished (within 5–ten min) and blood flows toward the lungs throughout the entire cardiac bicycle with the high basal flow during diastole being entirely due to the contribution of left-to-right shunting through the DA. However, although internet period through the DA is left-to-correct, instantaneous flow is mostly bidirectional, with right-to-left period occurring briefly during early systole simply then switching to left-to-right during late systole and throughout diastole ( Figure 3 ). This is thought to exist due to the anatomical relationship between the DA and pulmonary and aortic arteries (v). That is, during systole, the pressure wave exiting the right ventricle reaches the pulmonary artery/DA junction before the pressure wave exiting the left ventricle reaches the DA/aorta junction. As a result, briefly during early systole, the pressure at the pulmonary avenue/DA junction is greater than that at the DA/aorta junction resulting in a transient period of right-to-left shunting through the DA (23). However, when the systolic pressure wave from the left ventricle reaches the aortic end of the DA, the pressure gradient reverses resulting in a reversal inflow and left-to-right shunting through the DA.
Cardiovascular Changes with Delayed Umbilical Cord Clamping
Every bit indicated above, clamping the umbilical cord immediately later on nativity and earlier the lungs aerate causes a thirty–50% decrease in cardiac output due to an increment in afterload and a reduction in ventricular preload caused by the loss in umbilical venous return (25). This reduction in cardiac output persists as long as the filibuster between string clamping and the increase in PBF (induced by ventilation onset) continues ( Figure 4 ). Nonetheless, the decrease in ventricular preload can be avoided past increasing the PBF before the umbilical cord is clamped (25). Equally the increment in PBF results from lung aeration, establishing pulmonary ventilation while the umbilical cord is still intact allows PBF to increase while venous return and ventricular preload are maintained past umbilical venous return (25). Every bit a result, PBF tin can immediately take over the role of supplying ventricular preload before the string is clamped and umbilical venous return is lost. Thus, initiating ventilation before the cord is clamped can mitigate the changes in cardiac output, blood pressure, and cerebral claret flow acquired past umbilical cord clamping in preterm lambs ( Figure 4 ) (25).
The appropriate timing of umbilical cord clamping has been a topic of fence for millennia (26), even dating back to Aristotle in ~350 BC. The concept that umbilical cord clamping should not occur immediately after birth is oftentimes termed "delayed" cord clamping, although this implies that "immediate cord" clamping is the normal or natural timing for string clamping. This may not be right. Nevertheless, immediate string clamping after birth is the virtually commonly used and accepted practice worldwide and forms part of the current strategy for actively managing the tertiary stage of labor; the rationale for which is to reduce the take a chance of maternal hemorrhage (27). The active third-stage direction of labor includes oxytocin administration upon delivery of the infant'south anterior shoulder and immediate cord clamping (27). While it is established that active management reduces the risk of severe post-partum hemorrhage (PPH), it is focused on maternal outcomes and may overlook potential agin affects on the infant. Indeed, ane agin finding detailed in the Cochrane review showed that maternal oxytocin administration caused a meaning decrease in nascency weight (27), presumably due to a reduction in neonatal blood volume. As all infants had firsthand cord clamping (27), this finding is consequent with the view that delayed cord clamping causes placental to infant blood transfusion. There is also bear witness that oxytocin administration after commitment of the placenta is associated with less maternal blood loss than administration before delivery of the placenta (28) and that early administration of oxytocin may increase the run a risk of a retained placenta (29). Perchance, therefore, it is time to revisit how the 3rd phase of labor is managed with reference to the timing of oxytocin assistants and umbilical cord clamping and so that both maternal and neonatal outcomes are considered.
The available evidence, derived from both clinical and experimental studies, indicates that the timing of umbilical cord clamping can have significant consequences for the infant, with almost attention focusing on the potential for placental to babe blood transfusion (xxx,31). Indeed, it is possible that an increase in infant claret volume can business relationship for some of the cardiovascular benefits detailed above. Equally a result, the most recent (2010) guidelines from the International Liaison Committee for Resuscitation (ILCOR) have recommended that umbilical string clamping be delayed for at to the lowest degree 1 min in healthy term infants not requiring intervention (32). However, the optimal timing for the filibuster in cord clamping is not clear. In view of recent evidence, clamping the cord at a set arbitrary menstruum of time afterwards nascency with no reference to the infant's changing physiology would non appear to be physiologically sound or likely to optimize the potential benefits for an individual (25,26). The almost recent experimental evidence indicates that, rather than an arbitrary catamenia of time, the babe'southward respiratory function is probable to exist a better indicator for when the cord should exist clamped (25). That is, waiting until the infant has established effective animate, particularly if a pulse oximeter is used and showing an increasing oxygen saturation level, will ensure that PBF has increased and is able to provide sufficient venous return and preload for the left ventricle. Most infants will start animate and crying immediately after birth and, therefore, may receive little benefit from a one-min delay in cord clamping, unless it receives actress blood volume. On the other mitt, those infants who feel a delay in establishing animate later birth may receive substantial do good from delayed cord clamping (25), particularly if it allows time for spontaneous animate to commence. In any event, the mother may besides receive benefit from delayed cord clamping and administration of oxytocin after placental commitment (26).
In reference to the 2010 ILCOR guidelines, delayed umbilical cord clamping is only recommended for healthy term infants not requiring intervention (32). It has as well been suggested that delayed cord clamping is contraindicated for neonatal asphyxia and, instead, it is recommended that the infant should be immediately separated from the placenta and transferred for urgent resuscitation (33). However, information technology tin exist argued that delayed cord clamping would be of least benefit to infants not requiring intervention and of most benefit to those requiring respiratory back up immediately after nascency. Indeed, volume administration is an of import first step in the care of sick infants. Just it is too important to recognize that the benefits of delayed cord clamping in apneic asphyxic infants will probable depend on the crusade of the asphyxia. If information technology is due to cord compression or a placental complication, then it is unlikely that delaying cord clamping until after ventilation onset volition have any do good. Clearly, farther studies are required to define whether infants requiring resuscitation at birth, particularly preterm and asphyxic infants, would be improve served by receiving that support while notwithstanding attached to the umbilical cord.
Summary
Information technology is now well established that lung aeration, the increase in PBF, and maintenance of cardiac office in the newborn flow are intimately linked (5). More specifically, these major physiological events occur in a prepare interdependent sequence, beginning with lung aeration, which triggers an increment in PBF that then provides venous return and preload for the left ventricle (5). Equally left ventricular preload in the fetus is predominantly derived from umbilical venous return (two), if the period between umbilical cord clamping and breathing onset is delayed at birth, the infant will feel a menstruum of reduced cardiac function. This is due to a reduction in ventricular preload acquired past the loss of umbilical venous return and the disability of pulmonary venous return to supply sufficient preload before the lung aerates and PBF increases (25). If this is combined with a transient period of hypoxia, the infant is at run a risk of experiencing a hypoxic/ischemic upshot. Alternatively, if ventilation commences before the cord is clamped, there is no loss in preload as venous return immediately switches from umbilical to pulmonary venous return upon cord clamping. In view of this improved understanding of the inter-relationships between these major physiological events that underpin the transition to newborn life, the logic for delaying cord clamping until after animate has begun is now more readily credible.
Statement of Financial Support
This enquiry was supported past National Wellness and Medical Enquiry Quango (Commonwealth of australia) Program Grant (606789) and Research Fellowships (GRP: 1026890 and SBH: 545921), a Rebecca L. Cooper Medical Research Foundation (Australia) Fellowship (GRP), the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (Bethesda, Doctor, USA; Award Number R01HD072848), and a Financial Markets Foundation for Children (Commonwealth of australia) grant too as the Victorian government's Operational Infrastructure Back up Program (Commonwealth of australia).
Disclosure
The authors do not have whatsoever financial ties to products in the study or potential/perceived conflicts of interest to declare.
References
-
Hooper SB, Harding R. Fetal lung liquid: a major determinant of the growth and functional development of the fetal lung. Clin Exp Pharmacol Physiol 1995;22:235–47.
-
Rudolph AM. Fetal and neonatal pulmonary circulation. Annu Rev Physiol 1979;41:383–95.
-
Gao Y, Raj JU. Regulation of the pulmonary apportionment in the fetus and newborn. Physiol Rev 2010;xc:1291–335.
-
Hooper SB, Harding R. Office of aeration in the physiological adaptation of the lung to air-breathing at birth. Curr Respir Med Rev 2005;1:185–95.
-
Hooper SB, Siew ML, Kitchen MJ, te Pas AB. Establishing functional residual capacity in the non-breathing infant. Semin Fetal Neonatal Med 2013;18:336–43.
-
te Pas AB, Davis PG, Hooper SB, Morley CJ. From liquid to air: breathing subsequently birth. J Pediatr 2008;152:607–eleven.
-
Hooper SB, Kitchen MJ, Wallace MJ, et al. Imaging lung aeration and lung liquid clearance at birth. FASEB J 2007;21:3329–37.
-
Kitchen MJ, Lewis RA, Morgan MJ, et al. Dynamic measures of regional lung air volume using stage dissimilarity 10-ray imaging. Phys Med Biol 2008;53:6065–77.
-
Lewis RA, Yagi North, Kitchen MJ, et al. Dynamic imaging of the lungs using x-ray phase contrast. Phys Med Biol 2005;50:5031–40.
-
Bland RD, McMillan DD, Bressack MA, Dong 50. Clearance of liquid from lungs of newborn rabbits. J Appl Physiol Respir Environ Exerc Physiol 1980;49:171–vii.
-
Miserocchi Grand, Poskurica BH, Del Fabbro M. Pulmonary interstitial force per unit area in anesthetized paralyzed newborn rabbits. J Appl Physiol (1985) 1994;77:2260–8.
-
Hooper SB, te Pas AB, Lewis RA, Morley CJ. Establishing functional residual capacity at nativity. NeoReviews 2010;xi:474–83.
-
Fineman JR, Soifer SJ, Heymann MA. Regulation of pulmonary vascular tone in the perinatal period. Annu Rev Physiol 1995;57:115–34.
-
Teitel DF, Iwamoto HS, Rudolph AM. Changes in the pulmonary circulation during birth-related events. Pediatr Res 1990;27(iv Pt ane):372–8.
-
Hooper SB. Office of luminal volume changes in the increase in pulmonary blood flow at nativity in sheep. Exp Physiol 1998;83:833–42.
-
Moore P, Velvis H, Fineman JR, Soifer SJ, Heymann MA. EDRF inhibition attenuates the increase in pulmonary blood menstruation due to oxygen ventilation in fetal lambs. J Appl Physiol 1992;73:2151–7.
-
Dawes GS. Fetal and Neonatal Physiology. Chicago, IL: Year Book, 1968.
-
Fuhrman BP, Everitt J, Lock JE. Cardiopulmonary effects of unilateral airway pressure level changes in intact infant lambs. J Appl Physiol Respir Environ Exerc Physiol 1984;56:1439–48.
-
Fuhrman BP, Smith-Wright DL, Kulik TJ, Lock JE. Effects of static and fluctuating airway pressure on intact pulmonary circulation. J Appl Physiol 1986;60:114–22.
-
Lang JA, Pearson JT, te Pas AB, et al. Ventilation/perfusion mismatch during lung aeration at nativity. J Appl Physiol 2014;117:535–43.
-
Seed M, van Amerom JF, Yoo SJ, et al. Feasibility of quantification of the distribution of blood flow in the normal human being fetal circulation using CMR: a cross-sectional written report. J Cardiovasc Magn Reson 2012;14:79.
-
Polglase GR, Wallace MJ, Grant DA, Hooper SB. Influence of fetal breathing movements on pulmonary hemodynamics in fetal sheep. Pediatr Res 2004;56:932–8.
-
Crossley KJ, Allison BJ, Polglase GR, Morley CJ, Davis PG, Hooper SB. Dynamic changes in the direction of blood flow through the ductus arteriosus at nascency. J Physiol 2009;587(Pt 19):4695–704.
-
Grant DA, Hollander East, Skuza EM, Fauchère JC. Interactions between the right ventricle and pulmonary vasculature in the fetus. J Appl Physiol 1999;87:1637–43.
-
Bhatt S, Alison BJ, Wallace EM, et al. Delaying cord clamping until ventilation onset improves cardiovascular role at birth in preterm lambs. J Physiol 2013;591(Pt 8):2113–26.
-
Niermeyer S, Velaphi S. Promoting physiologic transition at birth: re-examining resuscitation and the timing of cord clamping. Semin Fetal Neonatal Med 2013;xviii:385–92.
-
Begley CM, Gyte GML, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews 2011;11:CD007412.
-
Huh WK, Chelmow D, Malone FD. A double-blinded, randomized controlled trial of oxytocin at the first versus the finish of the third phase of labor for prevention of postpartum hemorrhage. Gynecol Obstet Invest 2004;58:72–half dozen.
-
Jackson KW Jr, Allbert JR, Schemmer GK, Elliot M, Humphrey A, Taylor J. A randomized controlled trial comparing oxytocin administration before and later on placental commitment in the prevention of postpartum hemorrhage. Am J Obstet Gynecol 2001;185:873–7.
-
Yao Ac, Hirvensalo M, Lind J. Placental transfusion-rate and uterine contraction. Lancet 1968;1:380–3.
-
McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev 2013;7:CD004074.
-
Perlman JM, Wyllie J, Kattwinkel J, et al. Neonatal resuscitation: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care scientific discipline with treatment recommendations. Pediatr 2010;126:e1319–44.
-
Roehr CC, Hansmann G, Hoehn T, Bührer C. The 2010 Guidelines on Neonatal Resuscitation (AHA, ERC, ILCOR): similarities and differences–what progress has been made since 2005? Klin Padiatr 2011;223:299–307.
-
Kitchen MJ, Paganin D, Lewis RA, Yagi N, Uesugi Chiliad. Analysis of speckle patterns in phase-dissimilarity images of lung tissue. Nucl Instru Methods Phys Res 2005;548:240–half-dozen.
Author data
Affiliations
Corresponding writer
PowerPoint slides
Rights and permissions
Near this article
Cite this article
Hooper, Southward., te Pas, A., Lang, J. et al. Cardiovascular transition at nascence: a physiological sequence. Pediatr Res 77, 608–614 (2015). https://doi.org/10.1038/pr.2015.21
-
Received:
-
Accepted:
-
Published:
-
Consequence Date:
-
DOI : https://doi.org/10.1038/pr.2015.21
Further reading
Source: https://www.nature.com/articles/pr201521
0 Response to "What Major Changes Occur in the Circulation of Blood in the Heart of a Newborn Baby?"
Post a Comment